Quantitative image analysis has potential to transform the interpretation of bone marrow trephine (BMT) samples in myeloproliferative neoplasms (MPN) and improve the evaluation of anti-fibrotic therapies. To investigate the utility of recently developed algorithms evaluating reticulin fibrosis and megakaryocyte features in myelofibrosis (MF), we analysed samples from a multi-center, phase II study of zinpentraxin alfa (ZPN, PRM-151). ZPN is a recombinant form of human pentraxin-2 (PTX2) that has shown clinical activity as monotherapy and in combination with ruxolitinib (RUX) in a phase II trial in patients with Int-1/-2 or high risk MF (NCT01981850). In stage 2 of this study, patients ineligible for, intolerant of, or with an inadequate response to RUX were randomised to receive 0.3, 3.0, or 10.0 mg/kg ZPN on Days 1, 3, and 5 of Cycle 1, and every 4 weeks thereafter for up to 9 cycles. We demonstrate the potential of quantitative image analysis to augment and refine conventional expert histological assessment of MF.
Reticulin and H+E-stained BMTs from three timepoints (screening, C4D1 and C9D29) were analysed from 50/97 patients enrolled. Manual assessment (MA) of marrow fibrosis and megakaryocyte features was performed by blinded, independent central review. Assessment of fibrosis using the Continuous Indexing of Fibrosis (CIF) score and analysis of megakaryocytes was performed by automated analyses ( Ryou H, Leukaemia, 2022).
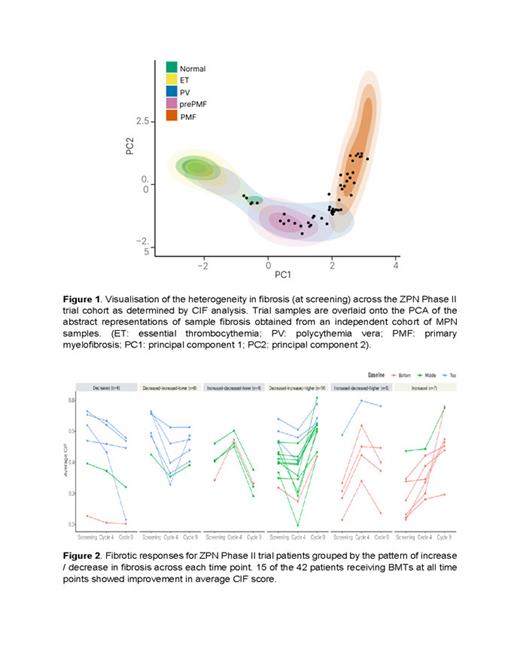
There was a moderate correlation between the automated average CIF score and manual fibrosis grade (Spearman's rho = 0.39). However, there was significant overlap in the distribution of CIF scores between samples manually assigned to MF-2 and MF-3. Approximately 38% of MF-2 samples fell within the interquartile range of CIF distribution observed in MF-3, and around 48% of MF-3 samples fell within the interquartile range observed in MF-2. Next, we visualised the screening BMTs in fibrosis PCA (principal component analysis) space to capture both average CIF score and Shannon entropy (‘unevenness‘) of CIF score for each sample. This revealed significant heterogeneity in reticulin fibrosis at screening when compared to an independent reference cohort of 130 MPN samples ( Ryou H, Leukaemia, 2022), likely reflecting the effects of pre-treatment [ Figure 1]. To track fibrosis across all timepoints we plotted the average CIF score for each sample. This revealed striking variability in fibrotic response within the trial cohort, with 15 of 42 patients (36%) demonstrating improvement in average CIF score [ Figure 2]. Notably, improvements in average CIF score by cycle 9 appeared to be most marked in patients with higher CIF scores at screening. No significant ZPN dose-dependent effect on fibrosis was observed.
We next measured megakaryocyte density (megakaryocytes per unit area of intertrabecular space) and compared it to MA. We found a strong correlation (Spearman's rho = 0.81) between automated and MA of megakaryocyte density, with no significant change across any of the treatment arms. For megakaryocyte clustering (defined as ≥ 3 megakaryocytes in direct contact) we observed a moderate to strong correlation (Spearman's rho = 0.62) between MA and automated assessment, with no significant change across any of the treatment arms. Finally, we sought to compare the sample megakaryocyte cell features (cytomorphology and topology) at screening with those of an independent reference cohort of 88 MPN samples ( Sirinukunwattana, Blood Adv., 2020). Strikingly, not only was there marked heterogeneity in megakaryocyte features between patient samples at screening, but the observed features were significantly different to those of newly diagnosed MF. No significant changes in megakaryocyte cytomorphology or topology were seen across any of the treatment arms.
In summary, our findings reveal considerable BMT morphological heterogeneity in patients participating in a Phase II trial of ZPN. This likely reflects poorly understood and under-recognised variation in morphological features encountered in patients with longstanding and/or pre-treated MF when compared to newly diagnosed patients. This highlights the potential of such variability to confound the evaluation of novel therapeutics in MPN, and emphasises the utility of robust quantitative methods to analyse and visualise morphological features in clinical study samples that can complement conventional manual assessment.
Disclosures
Sirinukunwattana:Ground Truth Labs Ltd.: Current Employment, Current equity holder in private company. Wood:Ground Truth Labs Ltd.: Current Employment. Aberdeen:Ground Truth Labs Ltd.: Current Employment, Current equity holder in private company. Rittscher:Ground Truth Labs Ltd.: Current equity holder in private company. Pozdnyakova:Sysmex: Consultancy; Scopio: Consultancy. Peale:Genentech: Current Employment, Current equity holder in publicly-traded company. Higgins:Genentech: Current Employment, Current equity holder in publicly-traded company; F. Hoffmann-La Roche: Current Employment, Current equity holder in publicly-traded company. Lundberg:F. Hoffmann-La Roche Ltd, Basel: Current Employment, Current equity holder in publicly-traded company. Trunzer:F. Hoffman-La Roche: Current Employment, Current equity holder in publicly-traded company. Harrison:GSK: Honoraria, Speakers Bureau; Galecto: Honoraria, Speakers Bureau; Abbvie: Honoraria, Speakers Bureau; AOP: Honoraria, Speakers Bureau; CTI: Honoraria, Speakers Bureau; Novartis: Honoraria, Research Funding, Speakers Bureau; BMS: Honoraria, Speakers Bureau; Morphosys: Honoraria, Speakers Bureau. Royston:Ground Truth Labs Ltd.: Consultancy.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal